Continuous Manufacturing
Applying JGC's Continuous Manufacturing Technology to Life Sciences
Continuous manufacturing is attracting considerable attention as a value-adding and growing trend in the manufacture of pharmaceuticals. Through mutually beneficial business exchanges with CONTINUUS Pharmaceuticals (CONTINUUS) in the US, the development of UT-01, a connected column-type flow synthesis unit, the investigation of continuous manufacturing technology for pharmaceuticals, the development of continuous anti-solvent crystallization technology, and continuous culture technology, JGC has brought together engineering technologies and expertise accumulated in the chemicals sector to contribute to the further streamlining of pharmaceuticals manufacturing through continuous manufacturing.
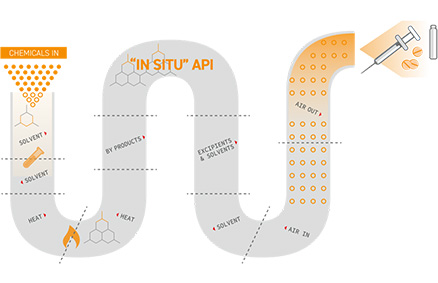
From Bulk Drugs to Pharmaceutical Preparations
- JGC aims to realize the continuous manufacturing of bulk drugs by adopting advanced manufacturing technologies, including those developed by CONTINUUS, enabling the continuous and seamless manufacture of pharmaceutical preparations.
-
Adopting CONTINUUS’ technology, a pilot plant is operating in Boston, Massachusetts.
- JGC and CONTINUUS collaborate in exploring opportunities for the advancement of pharmaceutical manufacturing in Japan.

Development of UT-01, a Connected Column-type Flow Synthesis Unit
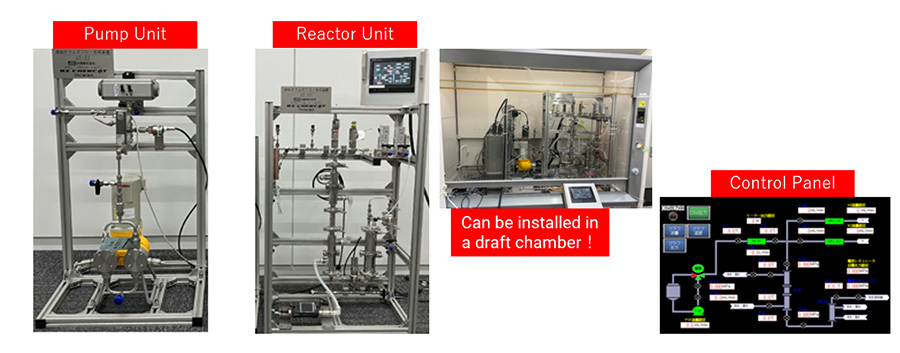
The UT-01 Connected Column-type Flow Synthesis Unit has been jointly developed by four companies and an institute (The University of Tokyo, TACMINA Corporation, Swagelok Japan, N.E. CHEMCAT Corporation, and JGC) at the Continuous Flow Research Center at the University of Tokyo. The bench-scale (reactor volume up to 1000mL) unit efficiently obtains data required for scale-up and small-scale prototyping. Adopting a divisible column, reaction rate analysis, multi-stage reactions, catalyst life analysis and reaction rate control are conducted. The Flow Unit is offered as a useful tool from scale-up to small-scale production.
Continuous Manufacturing Technology for Pharmaceuticals
Applying JGC's technologies in the chemical as well as life sciences sectors, the company has targeted the continuous manufacturing of pharmaceuticals, lowering of equipment costs, handling of wide-variety-small-lot production, and savings of space and manpower.
Furthermore, by maximizing the characteristics of compact continuous manufacturing equipment for solid preparations through plant modularization, construction time will be shortened and the company positioned to offer attractive proposals for modular plants as well as transform line organization.
Conducting technical research in the continuous manufacturing of pharmaceuticals, JGC shares its technology with equipment manufacturers not only in Japan but also in Europe and the United States.
Dependent on production volume, ingredients, and process conditions, the company proposes vertical or horizontal integration systems and optimal material handling as well as transportation methods.
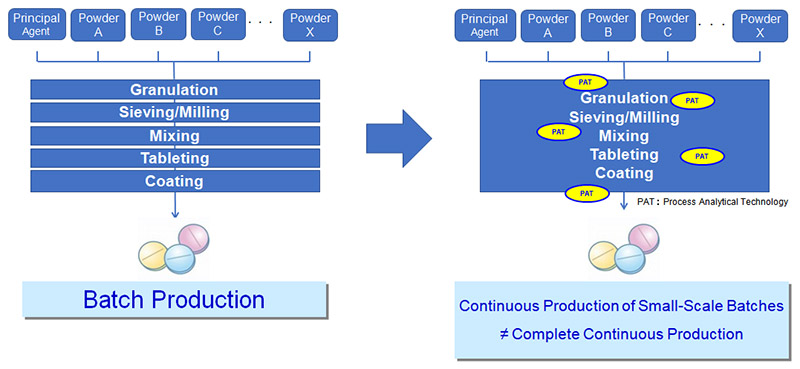
JGC gains a comprehensive understanding of the client’s process and proposes an optimized equipment/system design in consideration of discharge points in the system corresponding to each PAT shown in the following image, equipment design based on tablet properties including swelling after tableting, and evaluation of batch and continuous system designs for the coating process.
Continuous Manufacturing of Solid Preparations
Healthcare & Life Sciences
- Sterile Pharmaceutical Manufacturing Support Technology
- Single-Use Technology
- Design Technology for Active Ingredient Pharmaceutical Plants
- Logistics Planning Technology
- Containment Engineering
- Cell Culture Technology
- GMP Compliance Technology
- Continuous Manufacturing
- Layout Engineering
- Pharmaceutical Module Engineering
- Energy Saving/Environmental Technology
- Business Continuity Planning Technology
- Technologies of Swing and Nikki-Universal