High-Purity Hydrogen Sulfide Production Process

Hydrogen Sulfide (H2S) is widely used as raw material for synthesis of a number of sulfur-containing compounds.
JGC hydrogen sulfide production process produces high-purity (≧99.9 %) H2S by the reaction of hydrogen and sulfur. The production plant is compact and ensures easy and safe operation for the production of highly toxic hydrogen sulfide.
High-purity sodium hydrosulfide (NaSH aqueous solution) is also easily produced by the reaction between hydrogen sulfide and sodium hydroxide.
Schematic Flow Diagram of Production Units
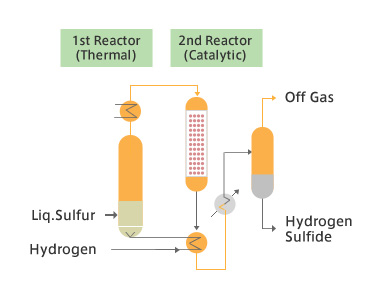

Features
- High-purity products
- Compact and economical production plants
- Lower cost and better safety than purchasing H2S
- High-performance and long-life catalyst for 2nd reactor
Applications
- Engineering plastic (PPS)
- Amino acid (methionine)
- Non-ferrous metal refining
- Pharmaceuticals
- Cosmetics
Experiences
- Hydrogen sulfide production : 2+1 (design in progress) units
- Sodium hydrosulfide production : 4+1 (design in progress) units
Award
- Technology Award of The Japan Petroleum Institute (2000)
Energy Transition - Gas/Oil/Chemicals
- The World's Most Advanced Energy-saving Condensate Desulfurization Process(JUSTTM Condensate)
- Integrated Hydrogen Desulfurization System - JUST® Refinery -
- Gas Oil Ultra-Deep Desulfurization Process
- Dimethyl Ether (DME) Synthesis Process
- High-Purity Hydrogen Sulfide Production Process
- DTP® Process
- Advanced Dehydration Process for Organic Compounds (Vapor-Phase PSA System)
- High-Throughput and High-Efficiency Extraction Column (WINTRAY®)