Life Science
Combining Innovation and Performance to Produce New Value - JGC Pharmaceutical Engineering
Through its "JGC Pharmaceutical Engineering", the company has been engaged in engineering in pharmaceuticals and life sciences for almost half a century.
Responding to rapid technological progress in the field of pharmaceuticals and R&D facilities, our more than 400 dedicated engineers continuously provide optimal solutions as well as precise, rapid responses based on our accumulated wealth of experience through over 600 projects successfully delivered.
JGC's Pharmaceutical Engineering Technology
JGC provides extensive technology in various fields including active ingredients, preparation, biotech, regenerative medicine, and research.
- Sterile Pharmaceutical Manufacturing Support Technology
- Single-Use Technology
- Design Technology for Active Ingredient Pharmaceutical Plants
- Logistics Planning Technology
- Containment Engineering
- Cell Culture Technology
- GMP Compliance Technology
- Continuous Manufacturing Technology
- Layout Engineering
- Pharmaceutical Module Engineering
- Energy Saving/Environmental Technology
- Business Continuity Planning Technology
- Technologies of Swing and Nikki-Universal
Engineers Who Cover Extensive Areas and Abundant Experience
In order to respond to all needs of pharmaceuticals and R&D facilities, JGC Group has many specialized engineers in a wide range of fields. Based on our excellent project management techniques, we achieve results that lead to customer satisfaction and trust by carrying out projects together with our customers.
Total Number of Engineers for JGC Group's Pharmaceutical-related Projects
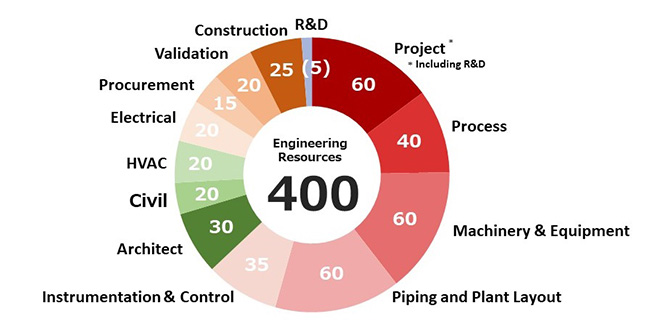
Japan's top mobilization capability
Wide-ranging engineers who cover everything from construction to machine equipment
JGC's Pharmaceuticals-related Project Experience
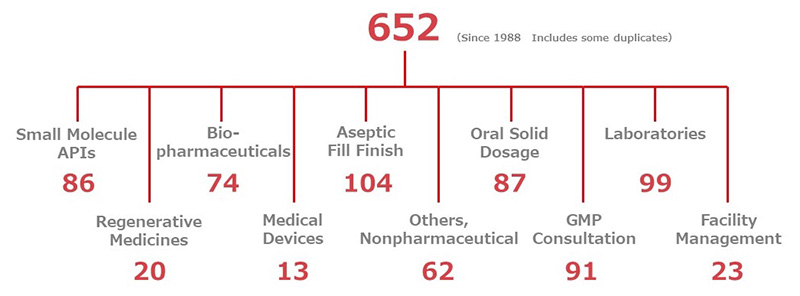
Featured Projects
To the Projects page
This introduces more experiences than published on the project page
Related Business Areas
Building New Hospitals through JGC's Project Management and Engineering Methods
Constant Search for New Advances Powers JGC Pharmaceutical Engineering
Supporting for improving the competitiveness of assembly factories